Clinical Trials
A clinical trial compares the effects of one treatment with another. It may involve patients, healthy people, or both.
From common myths to who you will meet along your clinical trial journey, this page aims to answer your questions. You can also use our clinical trial finder to look for a trial that is suitable for you.
Start by watching our short video for an overview of prostate cancer clinical trials.
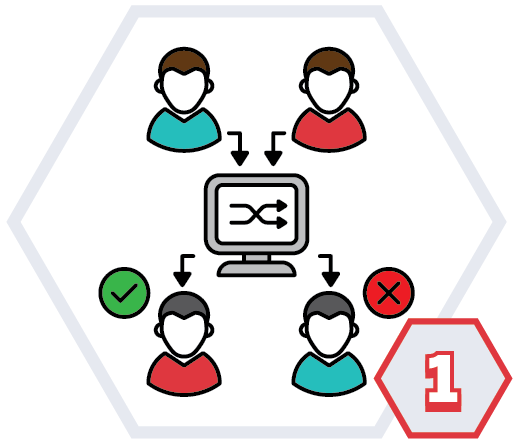
What are randomised control trials?
Randomised trials compare two or more different groups of patients. There is usually:
Randomisation is when people are put into either one of these groups by chance. A computer randomly decides which group you are in.
Randomisation is the best way of making sure that the results of trials are free from bias.

What are blinded trials?
What is standard of care treatment in a clinical trial?

What are eligibility criteria?


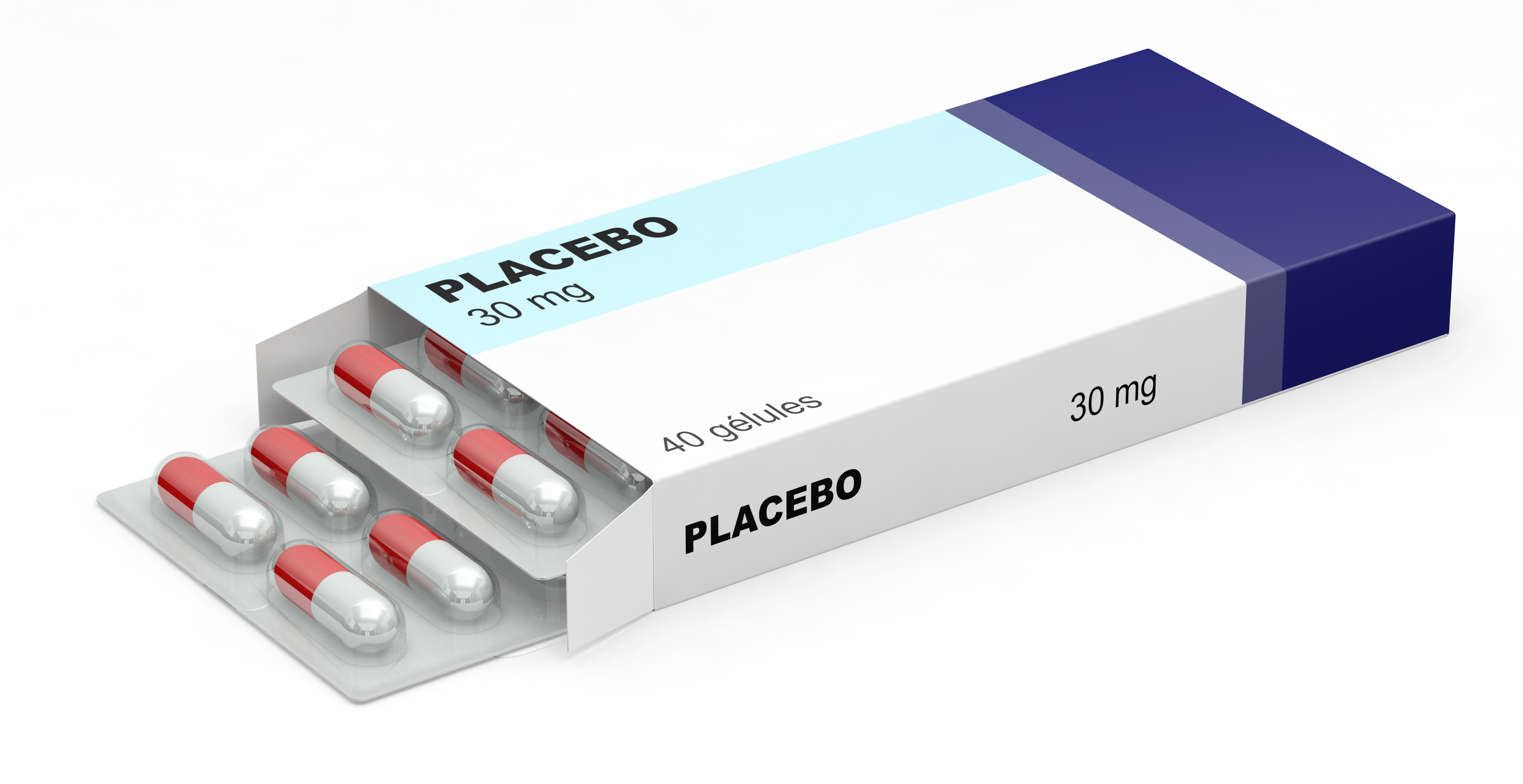
What is a placebo?

Will I get information about the trial before I make up my mind?


What is informed consent?

Take time to talk to your family, friends and healthcare team.

What is a research ethics committee (REC)?

What happens to tissue samples that are collected in a clinical trial?
for use in their research trial, they need to ask you to give your consent.
You will be given written information about what will happen to your tissue sample. Always read the consent form very closely before you sign it.
Who regulates tissue collection?
The Human Tissue Authority (HTA) are an arm’s length body of the Department of Health and Social Care. They:
You can find out more about their work by visiting their website: https://www.hta.gov.uk/

What happens at the end of the trial?

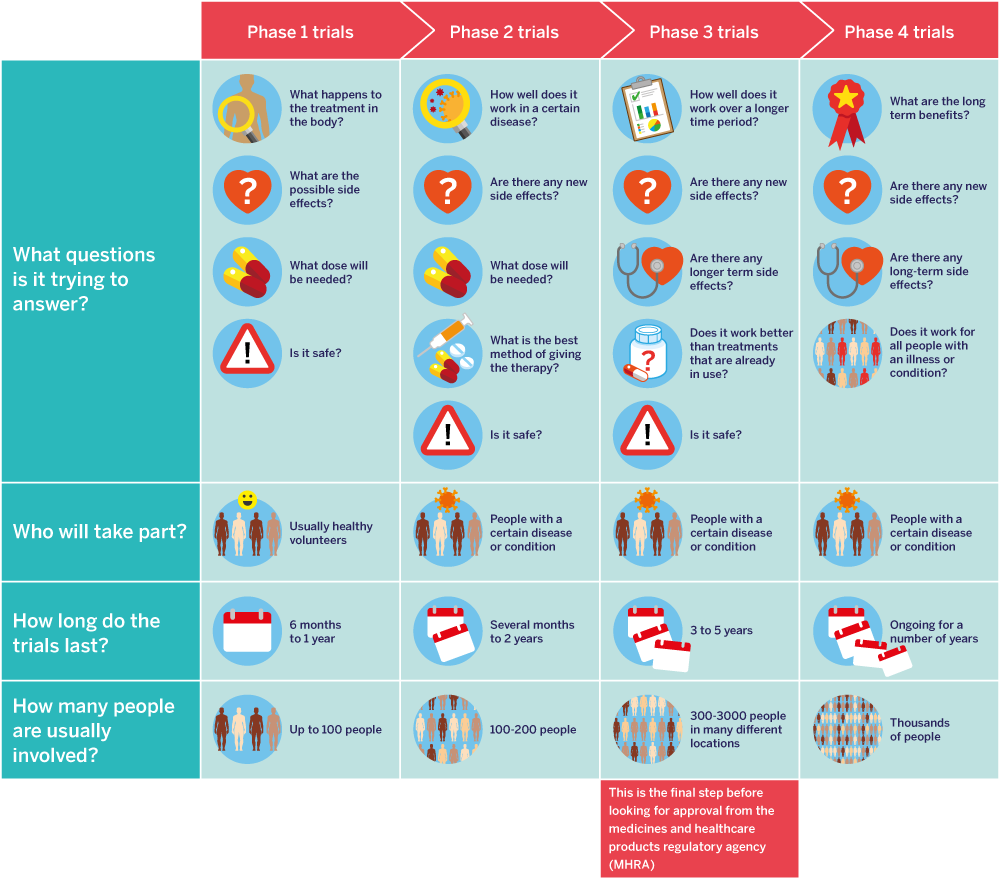
Clinical trials of new medicines go through four main stages or phases. These phases look at:
This infographic gives a simple overview of the four phases of clinical trials.



The journey through a clinical trial will be slightly different for each trial. In this next section, we will take you through a typical journey. The infographics show the steps you may go through if you decide you want to get involved in a clinical trial.
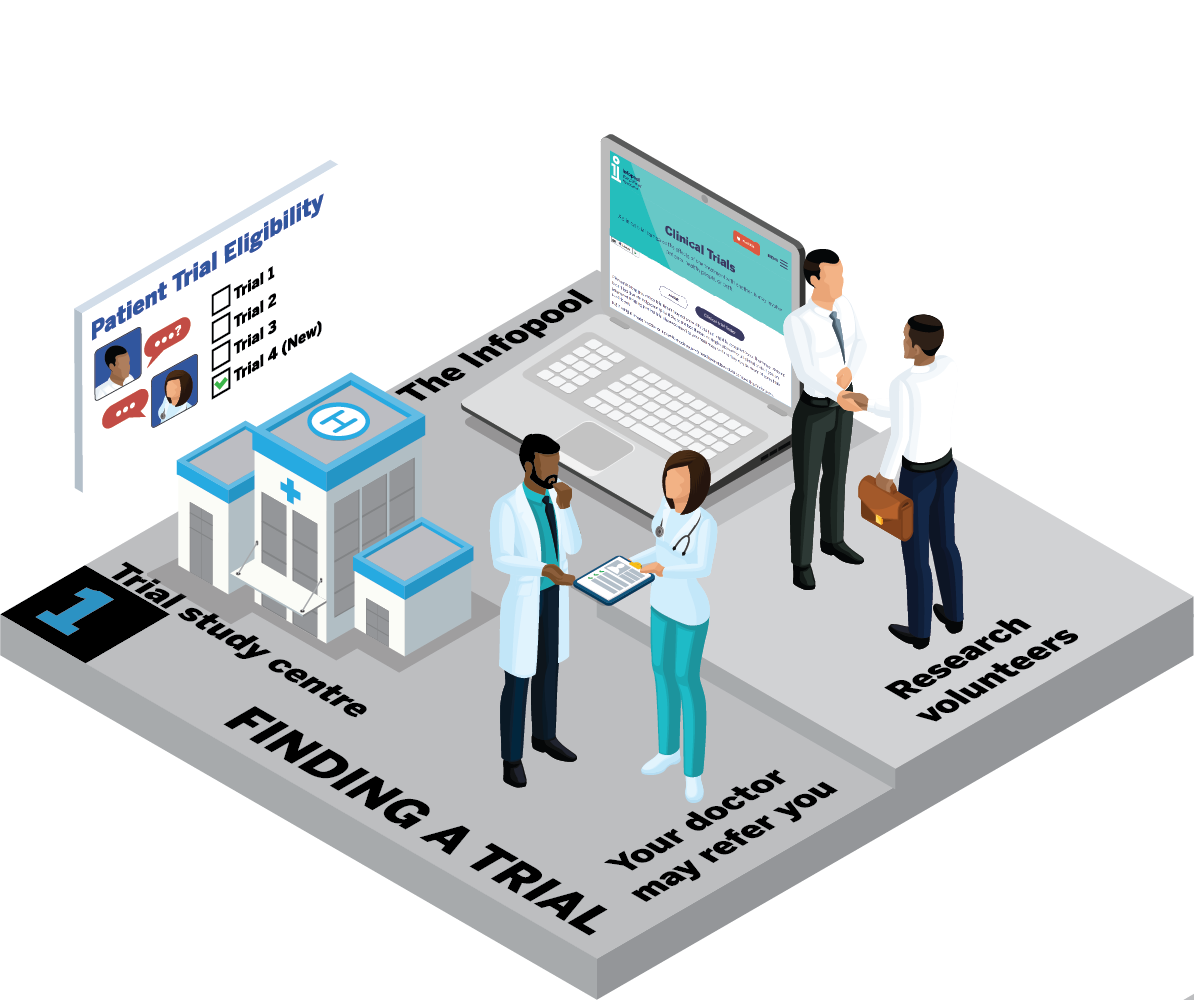
How can I find a clinical trial that's suitable for me?
Your journey begins with finding a clinical trial that's right for you. There are different ways to find these trials.
a. Your doctor may know of a trial suitable for you. They can make a referral to the trial team.
b. Your doctor may be involved with a clinical trial that is suitable for you. They will be able to give you more information.
c. You can explore clinical trial finders like the one on the Infopool website. If you find a trial that seems suitable for you, talk to your healthcare team for advice.
d. Consider becoming a research volunteer. It's a great way to learn about trials and be among the first to know about them. Ask your healthcare team for more information.
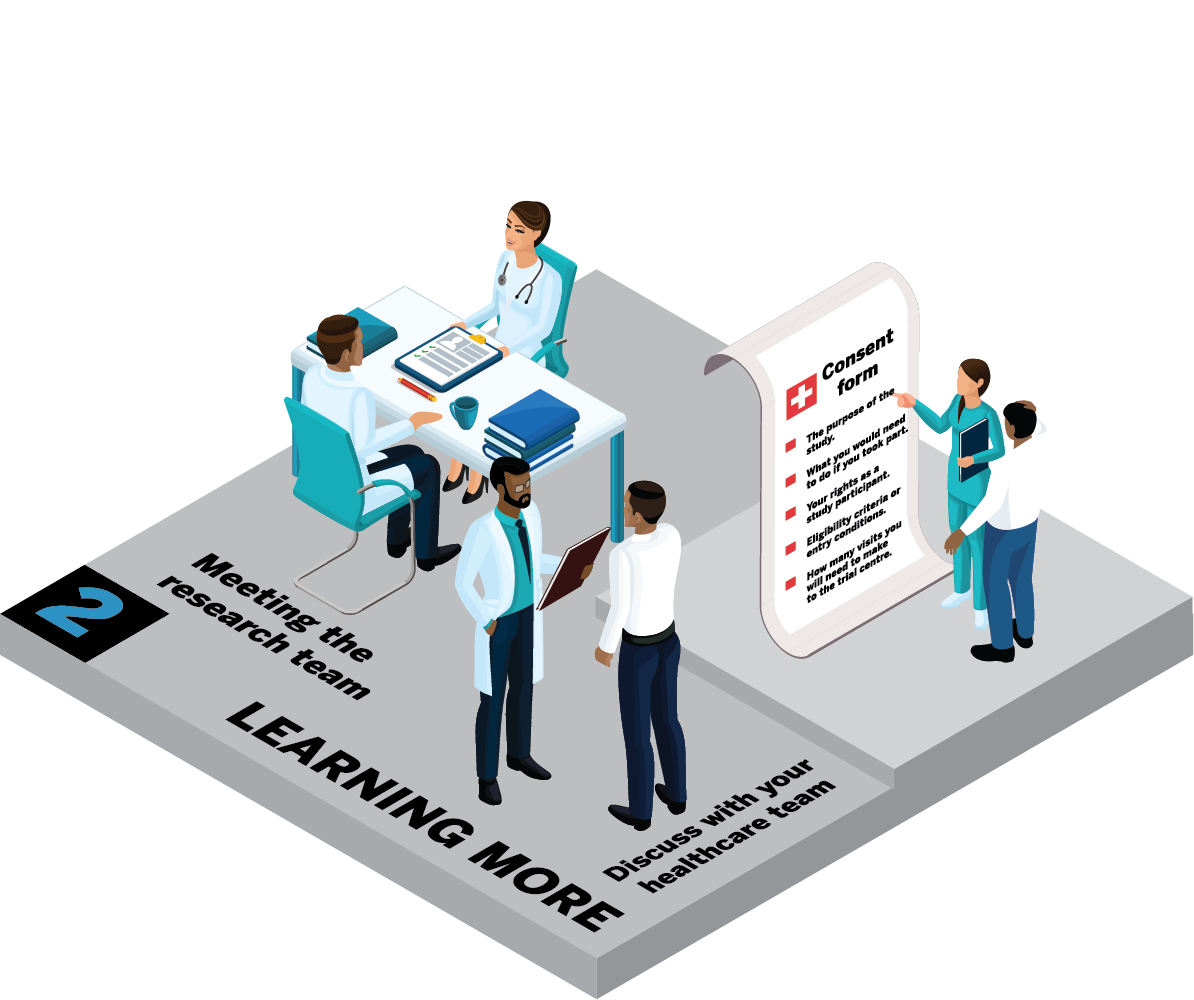
What information will I be given?
During this visit, you'll get detailed information about the study. This will involve guiding you through the informed consent. This will include things like:
Who can I talk to about the study?
You will get an information sheet to take home. This will give you the chance to talk it over with:
Remember:
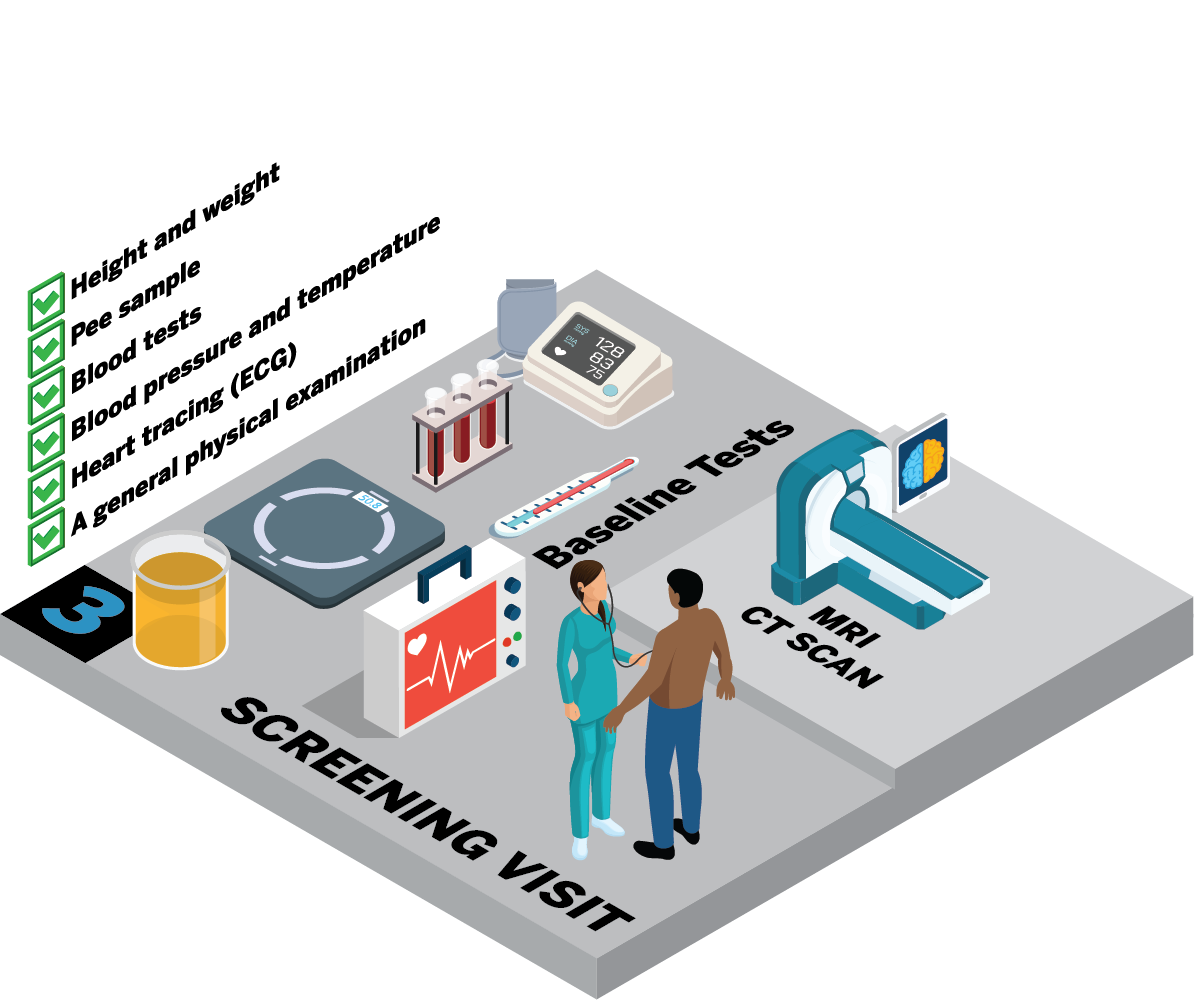
Will I need to have any tests?
Depending on the trial, you may need some extra tests to make sure you are:
The research nurse may do your:
You may also have a tracing of your heart (ECG). For some trials, you may need to have a CT or MRI scan.
When can I sign the consent form and start the study?
You can sign the consent form and begin your clinical trial journey. when you are:

What happens at the baseline visit?

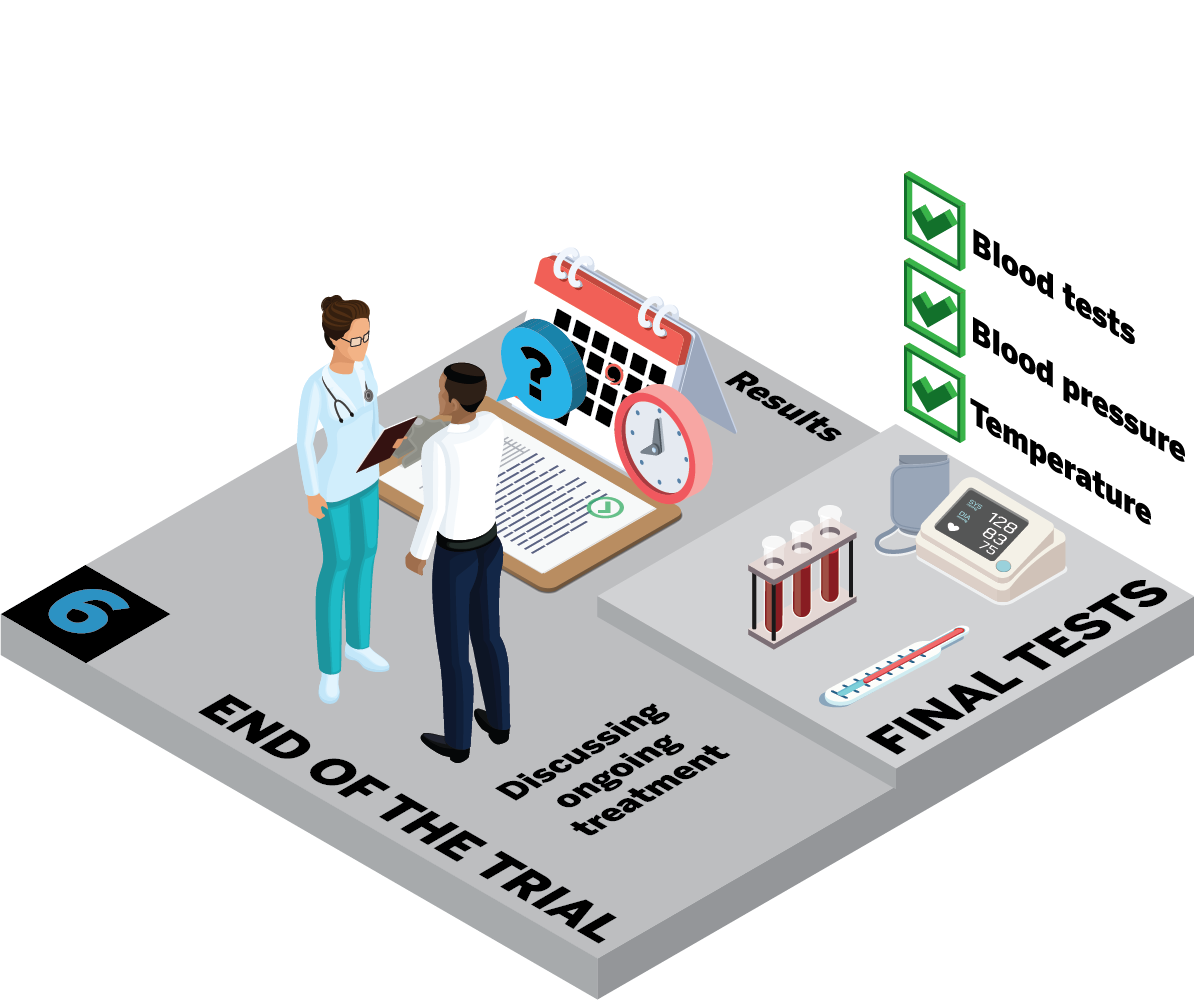
What happens at the final visits?
Can I carry on taking the study medicine?
What happens with my ongoing treatment?
Will I hear about the results of the study?
Are there any points I should remember?
How can I find out more about clinical trials?

The clinical trial toolkit is an information resource for people affected by prostate cancer. The toolkit development has been funded through an educational grant from Parexel.
Please note that this clinical trial finder may not show all trials that might be available to you. There may be some local trials that are not picked up by it due to the fact there is no single database for all clinical trials. If you are interested in taking part in a trial please do speak to your healthcare team as they may be aware of some trials local to you.
NB if using a private browser or incognito mode you may need to enable cookies to make this finder work.
You will hear many myths about clinical trials. This short animation aims to debunk the five most common myths.
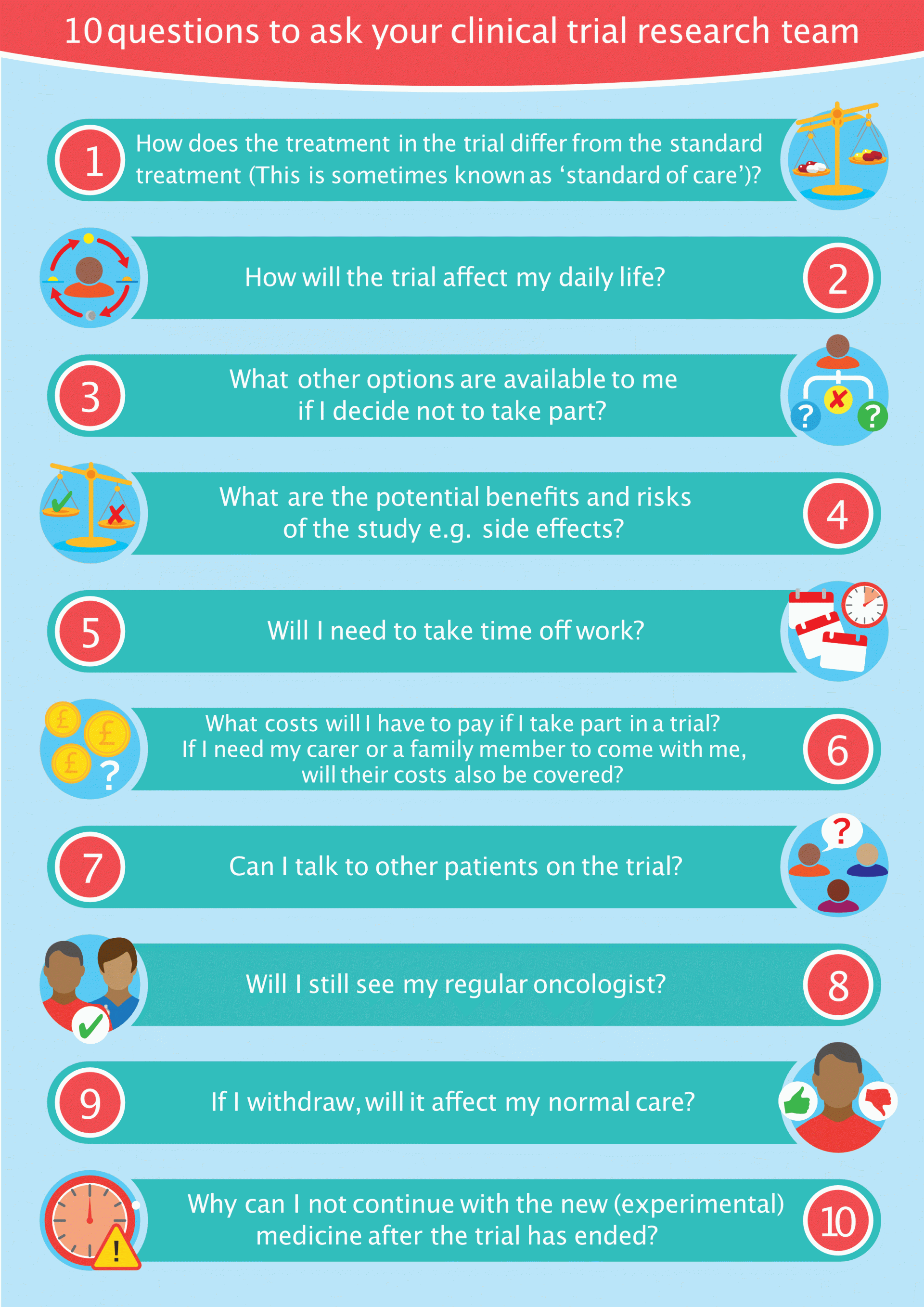
Here are 10 top questions that may not be covered in the informed consent.
Watch this short animation to learn more about the professionals you will meet if you decide to take part in a clinical trial.
Listen to patient Steve speak with senior research nurse Kelly about his experience of being part of a clinical trial.
He talks about:
The clinical trial toolkit is an information resource for people affected by prostate cancer. The toolkit development has been funded through an educational grant from Parexel.